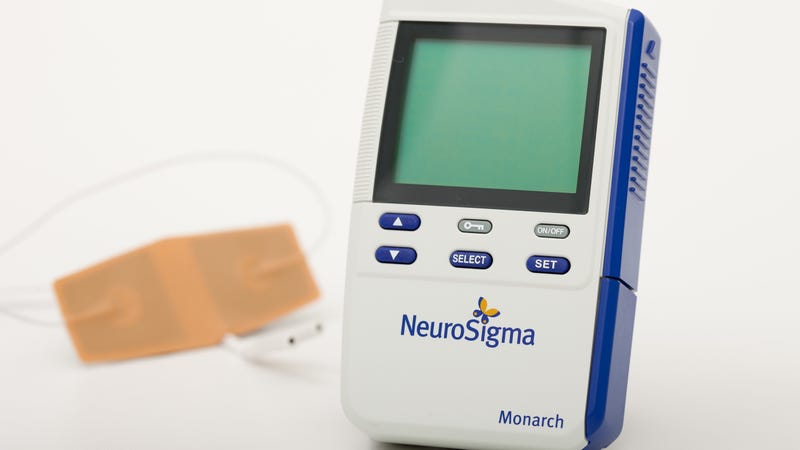
Families of teenagers with consideration deficit hyperactivity disorder (ADHD) will now beget a brand new, non-drug, therapy accessible to retain a watch on the condition: a medical blueprint linked to the forehead that sends light shocks to the apprehensive blueprint.
Over the weekend, the Meals and Drug Administration announced they granted clearance of NeuroSigma’s Monarch external Trigeminal Nerve Stimulation (eTNS) System for children with ADHD. (The FDA does no longer fundamentally “approve” medical devices the same methodology it does with capsules). The therapy will most likely be supposed for those ages 7 to 12 who’re no longer at the moment taking any utterly different prescribed capsules for his or her ADHD. But this may possibly also fully be accessible by strategy of prescription.
“This new blueprint presents a obtain, non-drug option for therapy of ADHD in pediatric sufferers by the utilize of sunshine nerve stimulation, a first of its sort,” Carlos Peña, director of the Division of Neurological and Physical Drugs Gadgets in the FDA’s Middle for Gadgets and Radiological Neatly being, acknowledged in a assertion.
There’s a large series of treatments that are traditional to lend a hand arrange ADHD in both teenagers and adults, an improved disorder that involves symptoms admire a fixed incapacity to listen to, impulsiveness, distress slumbering, and mood swings. They fluctuate from behavioral therapy to stimulants admire the note title Adderall and Ritalin to the selective norepinephrine reuptake inhibitor (SNRI) atomoxetine. These treatments all work to an extent, specifically stimulants, in that they’re higher than a placebo general. But no longer every person advantages from them.
As an illustration, about 20 to 25 percent of teenagers with ADHD don’t acknowledge to stimulants, in step with a 2016 overview. For these instances, atomoxetine is fundamentally effective, but it has its hold aspect-results, including brief suicide ideation. And even when stimulants lift out work, they’ll advance with severe aspect-results, including the long term possibility of dependancy and substance utilize disorder.
Nerve stimulation has emerged in present years as yet every other methodology to retain a watch on behavioral and neurological stipulations admire downhearted, epilepsy, and fright. There are a variety of forms of it—including one which requires implants surgically positioned into the mind—and we’re restful unclear about its actual mechanisms, but the mandatory gist is that nerve stimulation to the final note-attempting places can snappy composed the erratic mind exercise associated with a particular condition.
Within the case of the Monarch eTNS blueprint, the stimulation (which feels admire a tingling sensation on the pores and skin) is distributed from a exiguous, smartphone-sized blueprint to wires that are linked to a patch on the forehead ultimate-attempting above the eyebrows. The shocks then breeze alongside the trigeminal nerve, the cranial nerve that’s largely guilty for our sensations of biting and swallowing, which then straight or indirectly reach substances of the mind, admire the cerebral cortex, that lend a hand retain a watch on our emotions, behavior, and capability to heart of attention.
The major fragment of evidence traditional to persuade the FDA used to be a double-blinded, randomized and managed trial of 62 teenagers with moderate to severe ADHD, which used to be printed earlier this January. Those that got the real blueprint over a four-week length every night had a considerably higher discount of their symptoms (as judged by a clinician) than those given a non-functioning blueprint.
In step with the look’s authors, the stage of symptom improvement viewed in these teenagers used to be regarding the same you’d question to peek from those given any non-stimulant therapy, that are inclined to be moderately of much less effective than stimulants. But one actual lend a hand the Monarch looks to beget over stimulants is the shortage of any severe health risks. Aspect-results did embrace drowsiness, an develop in appetite, distress slumbering, tooth clenching, headaches, and fatigue.
Stable as the blueprint is, it’s supposed fully to be traditional with the supervision of a caretaker. The therapy also takes about four weeks sooner than symptoms reliably give a pick to. And children with pacemakers or utterly different devices implanted in the physique are no longer urged to place it to use.
Colin Kealey, vice president of Progressed Style & Scientific Affairs at NeuroSigma, told Gizmodo by strategy of electronic mail that there may be rarely all the time a particular date as of yet for the Monarch’s launch in the U.S., though they invent out hope to beget it accessible rapidly.
“We dwell up for the worth of the Monarch eTNS System will most likely be in-line with the present worth of note title ADHD drugs,” he added. “We stock out no longer dwell up for insurance protection protection in the early weeks after launch, alternatively, securing insurance protection as rapidly as capability is a high priority for NeuroSigma.”
The blueprint used to be already well-liked for the therapy of ADHD, epilepsy, and downhearted in the European Union, but this may possibly be its first recognition of any condition by the FDA.
“As a regular rule we lift out no longer touch upon particular plans, but NeuroSigma may possibly possibly also retain in thoughts operating extra scientific trials to make stronger submissions to the FDA for label enlargement and to search data from of clearance for a quantity of indications,” Kealey acknowledged.




Leave a comment
Sign in to post your comment or sign-up if you don't have any account.