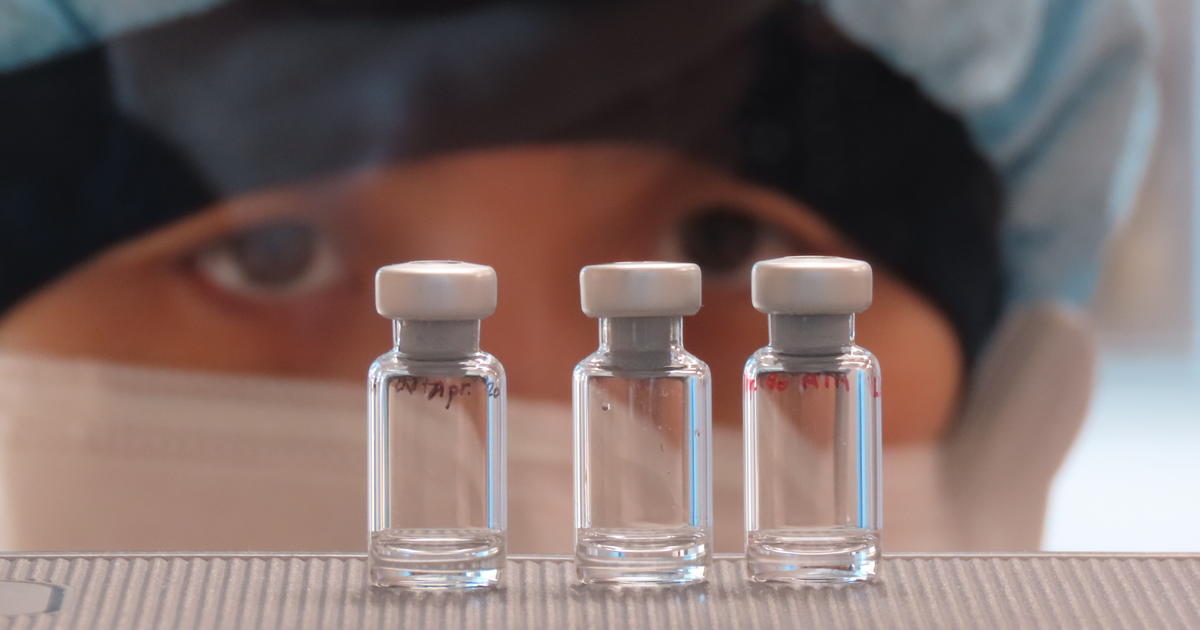
London — Within the realm high-tail for a COVID-19 vaccine, a seize option of human trials are now beneath plot, nonetheless it is scientists from England’s University of Oxford who appear most confident that they are onto a medication. Professor Sarah Gilbert heads the Oxford team in the aid of the capability vaccine being developed in partnership with the Jenner Institute. She’s talked about it has an “80% probability” of success, and it is seemingly to be available for large spend by the public as soon as September.
Human trials of the vaccine started Thursday in Oxford. This will seemingly be administered to 510 wholesome volunteers between the ages of 18 and 55.
U.Okay. Health Secretary Matt Hancock has talked about the authorities is “throwing the entirety” at efforts in the nation to tag a COVID-19 vaccine. He’s pledged around $25 million in public funding for the Oxford challenge and an extra $27 million to learn initiatives at Imperial College London. He says the U.Okay. is “on the entrance of the realm effort” to search out a vaccine.
Sean Elias/Oxford Vaccine Centre
“Now we maintain build more cash than any assorted nation real into a world see for a vaccine and, for the total efforts around the realm, two of the leading vaccine tendencies are taking assign right here at dwelling, at Oxford and Imperial,” Hancock talked about. “Each and each of these promising projects are making like a flash progress, and I’ve instructed the scientists leading them we are able to achieve the entirety in our energy to enhance.”
COVID-19, the illness precipitated by the unusual coronavirus first detected boring final yr in central China, has killed bigger than 184,000 contributors globally, and over 2.6 million maintain caught the virus.
World speed for a vaccine
Though there are 120 projects around the realm working in opposition to a vaccine, most attention-grabbing 5 were licensed for clinical trials on contributors.
As correctly as the Oxford initiative, a joint partnership between German biotech company BioNTech and U.S. pharmaceutical big Pfizer will originate trialing a doable vaccine later this month. This will seemingly be examined on 200 German volunteers former between 18 and 55.
The first human trial in the U.S. started in March. That vaccine is made by Moderna Inc. and is being administered on the Kaiser Permanente Washington Health Learn Institute in Seattle. Forty-5 wholesome adults, former 18 to 55, are enrolled in that trial, each of whom will receive two images, 28 days apart.
China moreover licensed its first human trial for a doable vaccine in March. Conducted by the militia-backed Academy of Navy Clinical Sciences and the Hong Kong-listed biotech firm CanSino Bio, researchers enthusiastic thunder the trial has already confirmed “sure outcomes.”
It be now not supreme vaccines that scientists are having a peek: Researchers are moreover trialing present capsules as most likely treatments for the unusual coronavirus illness, including long-relied on treatments for killer pathogens including Ebola, malaria and HIV.
Early outcomes were mixed, but till paunchy clinical trials are done and the suggestions analyzed, scientific doctors can not make sure that any of the medications are safe and effective.
Therapies apart, specialists thunder the realm will most attention-grabbing be ready to breathe a accurate enlighten of reduction when a vaccine is able to distribute broadly around the realm. Despite the self belief of the Oxford scientists and the fleet-monitoring of human vaccine trials, most specialists aloof mediate that can make a choice one other 12 to 18 months.



Leave a comment
Sign in to post your comment or sign-up if you don't have any account.