Hydrogen peroxide, a indispensable all-motive disinfectant, is repeat in most drugs cupboards within the developed world. But in distant villages in rising countries, the save it can maybe well additionally play a critical role in health and sanitation, it will be keen to reach by.
Now, a job developed at MIT might maybe maybe well additionally end result in a straightforward, inexpensive, portable instrument that might maybe maybe well additionally create hydrogen peroxide constantly from proper air, water, and electricity, providing a mode to sterilize wounds, meals-preparation surfaces, and even water provides.
The recent means is described this week within the journal Joule in a paper by MIT students Alexander Murray, Sahag Voskian, and Marcel Schreier and MIT professors T. Alan Hatton and Yogesh Surendranath.
Even at low concentrations, hydrogen peroxide is an efficient antibacterial agent, and after conducting its sterilizing feature it breaks down into horrid water, in distinction to various brokers such as chlorine that might maybe maybe move away undesirable byproducts from its manufacturing and use.
Hydrogen peroxide is proper water with an additional oxygen atom tacked on—it is H2O2, as a change of H2O. That additional oxygen is rather loosely skedaddle, making it a highly reactive chemical desirous to oxidize any various molecules around it. Or no longer it is miles so reactive that in high concentrations it will be used as rocket gas, and even concentrations of 35 p.c require very special handling and shipping procedures. The model used as a household disinfectant is mostly only 3 p.c hydrogen peroxide and 97 p.c water.
On account of high concentrations are keen to transport, and low concentrations, being largely water, are uneconomical to ship, the fabric is mostly keen to acquire in locations the save it will be in particular indispensable, such as distant communities with untreated water. (Micro organism in water provides will be effectively managed by including hydrogen peroxide.) As a end result, many compare groups across the realm were pursuing approaches to rising some originate of portable hydrogen peroxide manufacturing tools.
Many of the hydrogen peroxide produced within the industrialized world is made in substantial chemical plants, the save methane, or natural gas, is used to create a supply of hydrogen, which is then reacted with oxygen in a catalytic job below high warmth. This job is energy-intensive and no longer without anxiousness scalable, requiring substantial tools and a typical present of methane, so it does no longer lend itself to smaller items or distant locations.
“There is a rising neighborhood drawn to portable hydrogen peroxide,” Surendranath says, “on tale of of the appreciation that it can maybe well if truth be told meet heaps of wants, both on the financial facet moreover by human health and sanitation.”
Other processes developed to this level for presumably portable programs hold key limitations. As an illustration, most catalysts that promote the formation of hydrogen peroxide from hydrogen and oxygen also function heaps of water, main to low concentrations of the desired product. Moreover, processes that involve electrolysis, as this recent job does, in most cases hold a keen time isolating the produced hydrogen peroxide from the electrolyte fabric utilized within the job, once more main to low efficiency.
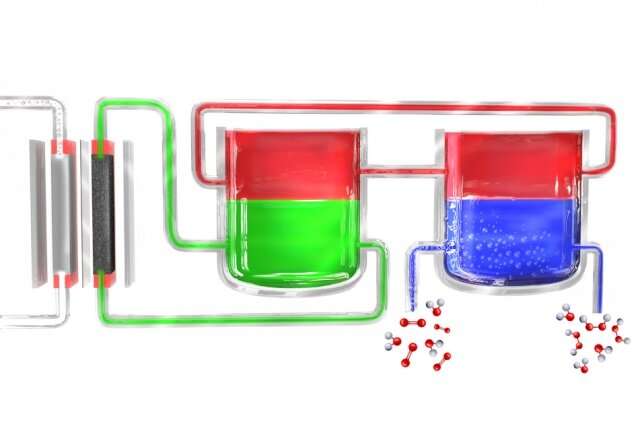
Surendranath and the the relaxation of the team solved the utter by breaking the job down into two separate steps. First, electricity (ideally from solar cells or windmills) is used to give plot water into hydrogen and oxygen, and the hydrogen then reacts with a “carrier” molecule. This molecule—a compound known as anthroquinone, in these preliminary experiments—is then launched proper into a separate response chamber the save it meets with oxygen taken from the surface air, and a pair of hydrogen atoms binds to an oxygen molecule (O2) to originate the hydrogen peroxide. In the job, the carrier molecule is restored to its normal articulate and returns to defend out the cycle at some stage in once more, so none of this fabric is consumed.
The job might maybe maybe well additionally address a form of challenges, Surendranath says, by making dapper water, first-aid fancy wounds, and sterile meals preparation surfaces extra available in locations the save they’re currently scarce or unavailable.
“Even at rather low concentrations, you perchance can additionally use it to disinfect water of microbial contaminants and various pathogens,” Surendranath says. And, he provides, “at increased concentrations, it will be used even to enact what’s known as developed oxidation,” the save in combination with UV light it will be used to decontaminate water of even stable industrial wastes, as an illustration from mining operations or hydraulic fracking.
So, as an illustration, a transportable hydrogen peroxide plant might maybe maybe well additionally simply be space up adjoining to a fracking or mining location and used to dapper up its effluent, then moved to one other space once operations terminate on the unusual location.
On this preliminary proof-of-thought unit, the concentration of hydrogen peroxide produced is peaceful low, nonetheless additional engineering of the system must peaceful end result in being ready to create extra concentrated output, Surendranath says. “One in every of the ideas to enact that’s to proper enlarge the concentration of the mediator, and happily, our mediator has already been utilized in move with the circulate batteries at if truth be told high concentrations, so we predict there is a route toward being ready to enlarge these concentrations,” he says.
“Or no longer it is model of an unprecedented job,” he says, “on tale of you build unparalleled things, water, air and electricity, that you just perchance can additionally supply within the community, and likewise you use it to function this critical chemical that you just perchance can additionally use to in point of fact dapper up the atmosphere and for sanitation and water quality.”
“The flexibility to produce a hydrogen peroxide resolution in water without electrolytes, salt, inappropriate, and heaps others., all of that are intrinsic to various electrochemical processes, is noteworthy,” says Shannon Stahl, a professor of chemistry on the College of Wisconsin, who was once no longer spicy on this work. Stahl provides that “Procure entry to to salt-free aqueous alternatives of H2O2 has extensive implications for colorful capabilities.”
Stahl says that “This work represents an modern software of ‘mediated electrolysis.’ Mediated electrochemistry provides a mode to merge extinct chemical processes with electrochemistry, and here’s an extremely compelling demonstration of this thought. … There are heaps of skill capabilities of this thought.”
Extra data:
“Electrosynthesis of hydrogen peroxide by piece-transfer catalysis.” Joule (2019). DOI: 10.1016/j.joule.2019.09.019
Journal data:
Joule
This tale is republished courtesy of MIT Data (web.mit.edu/newsoffice/), a standard location that covers news about MIT compare, innovation and teaching.
Citation:
Unusual job might maybe maybe well additionally function hydrogen peroxide available in distant locations (2019, October 24)
retrieved 24 October 2019
from https://phys.org/news/2019-10-hydrogen-peroxide-distant.html
This yarn is field to copyright. Rather than any dazzling dealing for the motive of inner most opinion or compare, no
phase might maybe maybe well additionally simply be reproduced without the written permission. The boom material is equipped for data capabilities only.





Leave a comment
Sign in to post your comment or sign-up if you don't have any account.