Scientists would possibly presumably sooner or later note the mysterious transition at the wait on of a century-veteran chemistry experiment. The facts of this transformation, in which adding electrons to a intellectual blue ammonia solution morphs it precise into a shining, steel bronze, maintain long eluded scientists.
The unique look displays the subtle tiny print of this swap, and displays that this transformation is leisurely, in desire to sudden. “What we’ve performed successfully is that we’ve slightly distinguished understood how these solutions behave at a huge fluctuate of concentrations the employ of a microjet technique,” acknowledged look co-author Ryan McMullen, a doctoral student in chemistry at the University of Southern California. This draw, which entails shooting hair-thin streams of the answer by a vacuum, has no longer been feeble on the shining liquid sooner than.
And the discovery would possibly presumably begin up unique forms of reactions in organic chemistry within the future, McMullen advised Stay science.
Related: 8 chemical parts you by no plan heard of
Metals are a diverse community. Some, treasure lithium, are light enough to float, while others, treasure lead or osmium are extraordinarily dense. Some require incredibly high temperatures to melt, while others soften without concerns (Mercury, to illustrate, melts at minus 38.3 levels Celsius, or minus 37.9 levels Fahrenheit). In the atomize, what metals maintain assuredly is their skill to conduct electricity at absolute zero, the point at which molecular circulation from warmth in actual fact halts.
However how kind some nonmetals remodel into metals? In a singular look, researchers answered that query by adding metals to liquid ammonia.
First, the researchers condensed ammonia, which is a fuel at room temperature, precise into a liquid by cooling it to damaging 27.4 F (minus 33 C). They then added both sodium, lithium or potassium, that are all alkali metals. (Rather famously, these metals react explosively when submerged in water.) The experiments were performed in collaboration with scientists from the Czech Academy of Sciences and the Fritz-Haber Institute of the Max Planck Society in Berlin, moreover to researchers in Japan and France.
Related: The tip 10 supreme explosions ever
The final end result used to be an expected response: The liquid ammonia pulled electrons from the steel. These electrons then grew to change into trapped between the ammonia molecules, creating the so-known as solvated electrons the researchers hoped to appear. At low concentrations, the dwell end result used to be a blue, non-steel liquid. Because the solvated, or trapped, electrons piled up, although, the answer transitioned to shining bronze.
The next field used to be to analyze how the solvated electrons behaved at completely different concentrations. This enthusiastic shooting a microjet of the answer — referring to the width of a human hair — by a beam of synchrotron X-rays, that are high-energy X-ray beams. The X-rays furious the solvated electrons, inflicting them to hop out of their liquid cage of ammonia molecules. The researchers would possibly presumably then measure how distinguished energy it took to release the solvated electrons.
The researchers realized that the larger the focus of solvated electrons, the more the pattern of energy release matched what’s viewed in a steel. Right here’s what which plan: Ought to you graph the amount of energy required to free electrons from their liquid ammonia cage, metals typically maintain what’s known as a “Fermi edge,” a extraordinarily abrupt transition, McMullen acknowledged. At decrease concentrations of solvated electrons, this energy-release graph appears more treasure a rounded hill. Most productive at bigger electron concentrations did this Fermi edge emerge. The brink displays how distinguished energy electrons maintain at a given temperature, McMullen added.
“Ought to you amplify the focus to the steel fluctuate then you definately search, this unprecedented pattern emerges that is terribly, very characteristic of a steel,” McMullen acknowledged.
The implications were intelligent due to the they showed that the steel-treasure liquid created by combining alkali metals and ammonia truly is a steel on a normal physical level, he acknowledged.
“It is an valid steel, it is far rarely one thing that simply appears treasure one,” McMullen acknowledged.
Lower-focus solvated electrons are feeble in a vogue of response known as a Birch response, which adds electrons to molecular constructions known as aromatic rings. This more or less response used to be feeble within the shape of the first oral contraceptive pills within the 1950s, McMullen acknowledged. By figuring out how solvated electrons work at high concentrations, researchers can doubtlessly come by unique forms of chemical reactions, he acknowledged. For example, they’d excite the solvated electrons with beams of light to come by them to behave in unique strategies.
“Ought to you tickle the electrons a diminutive so that they are more energetically furious, you may presumably presumably begin up looking out at some loopy reactions that would possibly presumably by no plan otherwise occur,” McMullen acknowledged.
The researchers reported their findings June 5 within the journal science.
At the delivery put published on Stay science.
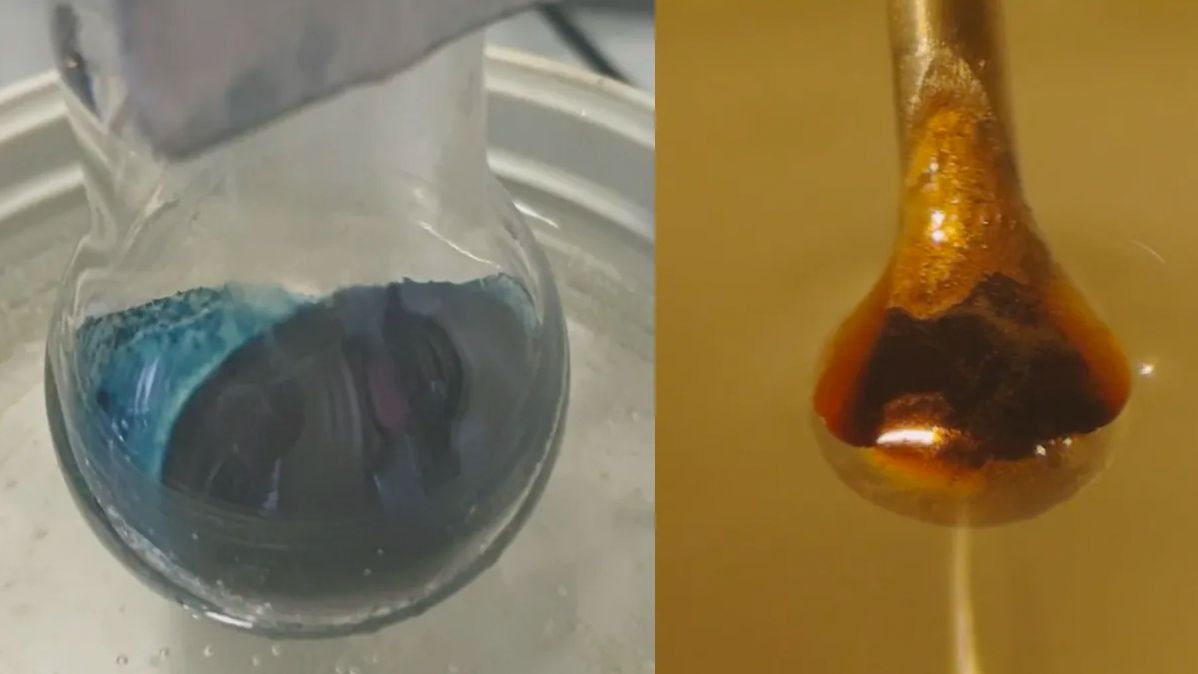




Leave a comment
Sign in to post your comment or sign-up if you don't have any account.